Chair And Boat Structure Of Cyclohexane
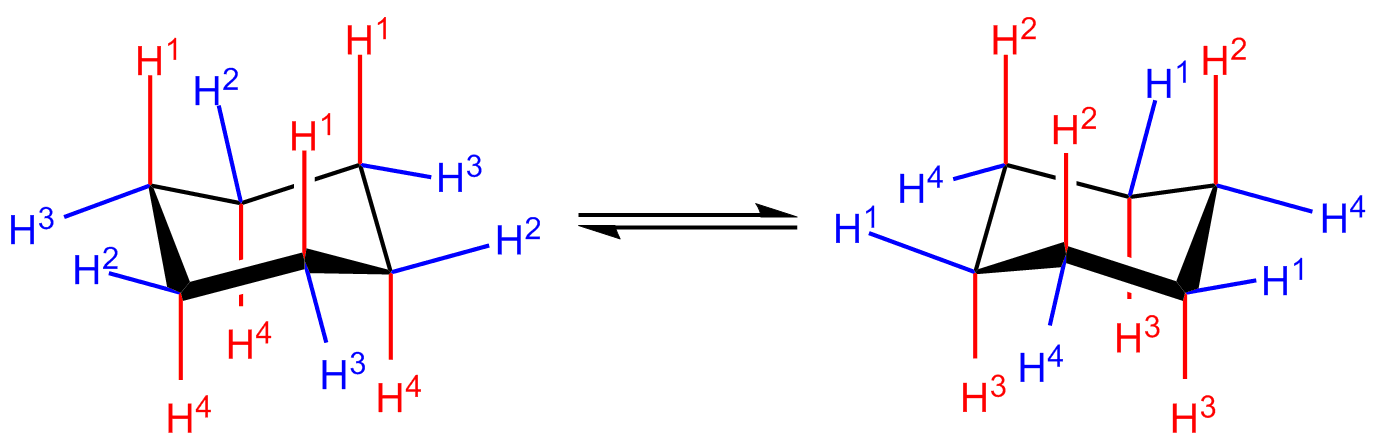
Chair and boat shapes for cyclohexane. chair and boat shapes for cyclohexane. so you kind of have this tetrahedral structure, and in the case of methane you have that 109.5 degree bond angles. carbon likes to form bonds of this shape. the different chair and boats, whether something is equatorial or axial can change if this were to flip. Drawing boat and chair conformations requires identifying the c-c bonds and the bonded substituents. the chair view is the more stable cyclohexane and the boat view is less stable, but both require 3-d representations of bent bonding patterns.. The chair, boat, and twist-boat conformations show the angles much closer to the ideal 109.5 o, and these are the shapes that most cyclohexane molecules are actually found to be in. the three.
We don't have to worry about any torsional strain, so the chair conformation is the most stable conformation for cyclohexane. next we'll take a look at the boat conformation. here we have the boat conformation of cyclohexane, if you look at the carbons it looks a little bit like a boat.. Chair-chair interconversion like other conformations we have studied, chair conformations are in a state of constant flux. because all the c-c bonds are interconnected, they cannot rotate independently but have to move together. for example, one end of the chair could "flip up" to put the cyclohexane ring in a boat conformation.. This is the predominant structure adopted by molecules of cyclohexane. an energy diagram for these conformational interconversions is drawn below. the activation energy for the chair-chair conversion is due chiefly to a high energy twist-chair form (tc), in which significant angle and eclipsing strain are present..